Deepening Technical Understanding of Pigments and Color Principles
On January 9, 2026, Fineland Chem organized a professional internal training session focused on the theoretical knowledge of pigments and color science. The session offered a detailed exploration of the micro-level mechanisms behind color, pigment properties, and application principles, aiming to strengthen employees’ understanding of pigment chemistry, structure, and performance.
The training was designed to help both new and experienced team members gain a solid technical foundation for their work in pigment sales, formulation support, and product development.
The Three Elements of Color
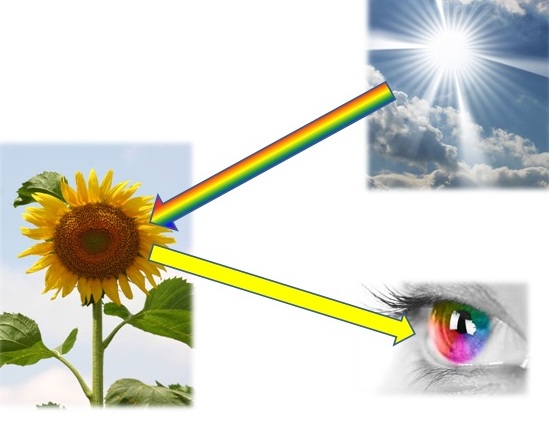
Color is not an inherent property of an object—it is the result of interaction between light, the object, and the observer.
Light – Light is a form of electromagnetic radiation. The visible light spectrum (approximately 380–780 nm) determines what we perceive as color. When white light hits a surface, part of it is absorbed and part is reflected. The reflected wavelengths determine the perceived color.
-
Object – The object’s surface determines how it absorbs and reflects specific wavelengths of light. Pigments control this behavior by selectively absorbing and scattering light.
-
Observer – The human eye and brain interpret the reflected light. Our eyes contain cone cells that are sensitive to red, green, and blue light, allowing us to distinguish millions of color variations.
In addition, color can be quantified by three measurable attributes:
-
Lightness (L) – Represents the brightness or darkness of a color.
-
Chroma (C) – Indicates color purity or saturation (vividness).
-
Hue (H) – Defines the color family, such as red, yellow, or blue.
Mechanism of Pigment Color Change and Color Difference
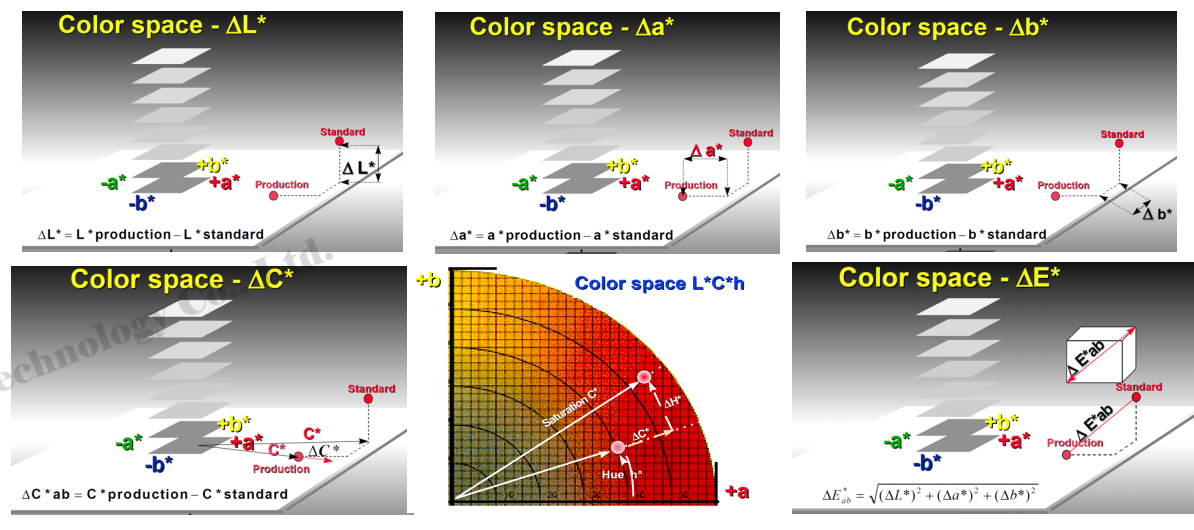
Pigment color is not static. It may vary slightly depending on particle size, crystal form, surface treatment, and the medium in which it is dispersed. These differences can be measured and quantified using color science methods.
Color difference (ΔE) quantifies the deviation between two colors. It is calculated using colorimetric equations based on measured lightness, chroma, and hue differences. A smaller ΔE value indicates a closer color match.
Color difference measurements are essential for color quality control, formula adjustment, and batch consistency in pigment production.
Pigments vs. Dyes
One of the most fundamental distinctions in colorants is between pigments and dyes:
| Property | Pigments | Dyes |
|---|---|---|
| Solubility | Insoluble, exist as solid particles suspended in a medium | Soluble, dissolve completely in the medium |
| Transparency | Generally opaque | Highly transparent |
| Application | Require dispersion to achieve even color | Dissolve directly in the solvent |
| Durability | High lightfastness and weather resistance | Lower resistance to light and chemicals |
| Usage | Used in coatings, plastics, and inks | Used in textiles, leather, and paper |
Pigments provide long-term stability and color retention, which is why they are preferred for industrial coatings, plastics, and automotive applications, where durability is crucial.
Classification of Pigments
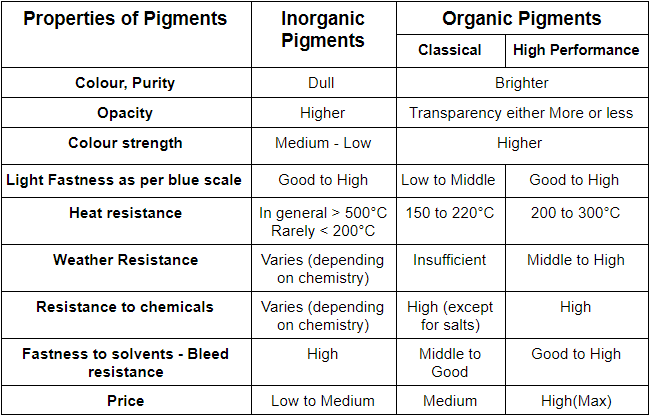
From a chemical composition perspective, pigments are broadly categorized into:
-
Inorganic Pigments – Examples include titanium dioxide, iron oxides, chromium yellow, molybdate orange, chrome green, ultramarine, and bismuth vanadate. They typically offer high opacity, excellent weather resistance, and easy dispersibility.
-
Organic Pigments – Examples include phthalocyanine blue, azo red, quinacridone, and DPP (diketopyrrolopyrrole) red pigments. They are known for brighter colors and higher chroma, but require more complex dispersion.
-
Effect Pigments – Include metallic pigments (aluminum paste, bronze powder), pearlescent pigments, fluorescent and phosphorescent pigments, and molybdenum disulfide-based special-effect pigments.
Important and Special Organic Pigments
Pigment Red 177 (PR177)
A high-performance anthraquinone-based red pigment, PR177 offers excellent lightfastness, heat stability, and solvent resistance. It produces a deep bluish-red shade and is widely used in automotive coatings, industrial paints, and high-end plastics.
[Image Alt Text: “Pie chart showing application distribution of PR177 pigment across coatings and plastics”]
Pigment Red 254 (PR254)
PR254, part of the Diketopyrrolopyrrole (DPP) pigment family, provides brilliant red color, high tinting strength, and exceptional durability. It is a signature pigment for premium automotive coatings, industrial paints, and plastics.
Pigment Blue 60 (PB60)
PB60 is a perylene blue pigment with excellent chemical resistance and lightfastness, providing deep, transparent blue shades. It is mainly used in automotive coatings, high-performance plastics, and ink formulations.
Pigment Violet 23 (PV23)
PV23 is a dioxazine violet pigment featuring a bluish-violet tone, high tinting strength, and excellent light and weather resistance. It is commonly used in automotive coatings, industrial paints, plastics, and inks for its strong color intensity and durability.
Pigment Blue 15:6 (PB15:6)
PB15:6 is a phthalocyanine blue pigment with a bright reddish-blue shade, excellent stability, and UV resistance. It is widely applied in coatings, plastics, and printing inks, offering strong color strength and good dispersibility.
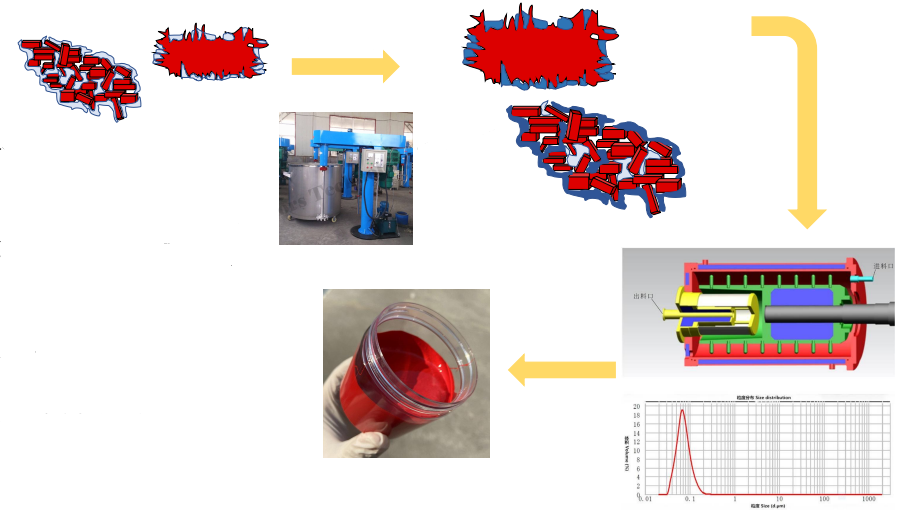
Dispersion of Organic Pigments
The dispersion process determines the final performance of pigments in a formulation. Pigments exist in three physical forms: single crystals, aggregates, and agglomerates. Proper dispersion breaks down these structures to achieve uniform color strength, gloss, and stability.
The dispersion process includes four main stages:
-
Wetting – The pigment surface is wetted by the resin or solvent, reducing surface tension and improving interaction.
-
Breaking Down (Deagglomeration) – Shear forces break large agglomerates into smaller aggregates or primary particles, improving color development.
-
Dispersion – Pigment particles are evenly distributed throughout the medium. Viscosity and pigment concentration are key factors.
-
Stabilization – Dispersants adsorb onto pigment surfaces, preventing re-agglomeration and maintaining a stable suspension.
Each stage influences the pigment’s final performance in terms of color strength, gloss, and stability.
Summary
Through this pigment theory training session, participants gained a deeper understanding of how pigments create color, how they differ from dyes, and how different pigment structures influence performance.
By mastering these concepts, Fineland Chem team members are better prepared to communicate technical knowledge with customers, assist in formulation troubleshooting, and maintain high-quality standards in pigment applications.
As Your Reliable Pigment Partner, Fineland Chem will continue to provide ongoing internal training to ensure that every team member has a strong foundation in both pigment science and practical applications.